Oil Drillers, Environmentalists Agree on Small, Sensitive Spectrometer
Subheadline
Tiny tool to study water quality gets results anywhere
At a mere 93 million miles from the Sun, Earth really shouldn’t have as much water as it does. No one knows how it all got here. Max Coleman of NASA’s Jet Propulsion Laboratory in Southern California subscribes to the theory that a significant amount of water arrived on comets that bombarded the planet close to 4 billion years ago. To look for evidence, he wants to examine water from as many locations around the solar system as possible.
Specifically, he would need to discover the ratios of different isotopes within those water samples. These are slightly different versions of the water molecule, and the proportions in which they’re present can tell researchers a lot about a water sample’s history.
A former geochemical researcher for the oil and gas industry, Coleman also knew isotope ratios would hold valuable information for offshore drilling operations, environmental researchers, and others here on Earth.
In 2012, NASA sent a tunable laser spectrometer to analyze isotopes on Mars aboard the Curiosity rover. There, the instrument analyzed hydrogen, carbon, and oxygen isotopes in the atmosphere and in gases released from samples the rover acquired.
Now a smaller, simpler commercial version is available to analyze Earth environments.
Draw Down the Volume
Isotopes are atoms of the same element that have different numbers of neutrons, giving them different weights, although they’re chemically identical. These atoms can combine with others to form molecules that are also identical except for their weight. The ratio of heavy to light molecules in a sample depends on processes it underwent in the past, from evaporation and condensation to biological metabolism. But counting the molecules in a sample and identifying their ratios is no easy task.
Groundbreaking though it was, Curiosity’s instrument has to fill a gas sample cell that’s almost a quart in volume to get an accurate reading, which makes it bulky and, for certain applications, useless. Now, following years of work with JPL, as well as the Department of Energy and the National Oceanic and Atmospheric Administration (NOAA), a company called Guiding Photonics LLC of Torrance, California, has produced a laser spectrometer that has similar accuracy but requires a sample volume of less than a quarter teaspoon.
Guiding Photonics, which produces commercial fiber optics and sensors, recently spun off from OKSI, formerly known as OptoKnowledge Systems Inc., a research and development company with a long history of working with NASA. Starting in 2014, OKSI received two Small Business Technology Transfer (STTR) contracts through JPL to develop a laser spectrometer for sensing trace gases and isotopes.
Like the version on Curiosity, the spectrometer directs laser light through a gas sample. Molecules in that sample vibrate and rotate at different frequencies depending on which isotopes of which element are present, causing them to absorb different wavelengths of light. By observing how much certain wavelengths are absorbed, the device can identify molecules and their isotopes.
While the version on Mars bounces that laser light back and forth more than 80 times through a cylindrical cell to maximize absorption, the prototype OKSI built with STTR funding instead sent the laser through a long, thin optical fiber with a hollow, reflectively coated core. The design, which the company calls a capillary absorption spectrometer, requires only a tiny gas sample to fill the fiber, while its length still provides plenty of opportunity for laser photons to be absorbed by the sample molecules. The fiber cable can also be coiled to produce a compact device.
A team at a Department of Energy lab first put forward the concept, and the device OKSI built with NASA STTR funding proved it worked. In addition to funding, the company also used previous NASA research to build its first capillary absorption spectrometer. “They did a lot of pioneering work – simple things like, what part of the molecular fingerprint of methane we should target,” said Jason Kriesel, lead scientist at OKSI and president of Guiding Photonics. “When they put together that system for the Curiosity rover, they did a lot of basic research that we can build on.”
Coleman got involved in the 2014 project when he realized it was “really up my street,” he said, and when that funding ran out, he and OKSI collaborated on a project with NOAA. Researchers there were interested in using such a device to test methane from deep-sea vents, and a Space Act Agreement let JPL help OKSI build on its earlier prototype under grants from NOAA.
Using the spectrometer underwater required gases to be extracted from a water sample. This has always been accomplished with a membrane, which both throws off isotope counts and requires a bulky device, said Kriesel. Instead, Coleman came up with the novel solution of incorporating a small, depressurized chamber at the intake to the fiber-optic sample cell. The low pressure causes gases to bubble out from the water, making them available for testing.
“This degassing approach wasn’t used before because it would be difficult to fill up a normal cell with the small amount of gas you get from it,” said Kriesel. “But since you have this small hollow-fiber cell, you can get away with this new method.”
Guiding Photonics released its Omega fiber-optic gas cell for laser spectroscopy in 2021, available with or without accompanying sensors and degassing chamber.
Striking Oil, Preserving the Environment
Most of the early commercial interest in the technology has come from the offshore oil and gas industry, where at least one major supplier has already bought multiple systems and others are showing interest, Kriesel said. He noted that offshore oil and gas drillers have a few reasons to look at, for example, methane isotopes. Methane might indicate an underground oil deposit, or it might be coming from a decaying whale carcass – isotopes can reveal the difference. Characterizing methane content of the water in an area before drilling also informs environmental impact statements, and it allows later testing during operations to reveal any leaks, helping with facility maintenance and minimizing environmental damage.
Normally, Kriesel said, the driller would have to send the samples to a lab and await results, which would direct the next round of sample collection. Instead, getting immediate results lets the user characterize an entire area in one attempt. “This technology gives them the ability to measure in the field and make decisions in real time, in terms of what they want to study further, which can save them a lot of money,” he said.
Other pilot projects have used the new capillary absorption spectrometer to look for methane in coastal wetlands – which would help climate researchers understand sources and sinks of greenhouse gases – and to monitor emissions from prescribed forest burns using a drone. Being small helps the device fit on a drone, where it can measure carbon dioxide-to-carbon monoxide ratios, imparting valuable information about the fire below.
OKSI is now working under SBIR funding from NASA’s Goddard Space Flight Center in Greenbelt, Maryland, to develop an instrument that could support Coleman’s search for the origins of water on Earth. Kriesel said he expects the project – adapting the technology to examine water isotopes on the Moon – to also result in a commercial water isotope analyzer from Guiding Photonics.
“For my interest in water,” said Coleman, “this could become a very small instrument that could take advantage of other NASA missions to go here, there, and everywhere, studying water around the solar system.”

Operators with the National Oceanic and Atmospheric Administration (NOAA) watch from a control room as a remotely operated vehicle measures methane concentration and isotope ratios at an underwater volcano off the coast of Oregon in July of 2022. It was the first deep-water test of the capillary absorption spectrometer developed by Optoknowledge Systems Inc. (OKSI) with funding from NOAA and NASA and now commercialized by Guiding Photonics. Credit: Andrew Fahrland
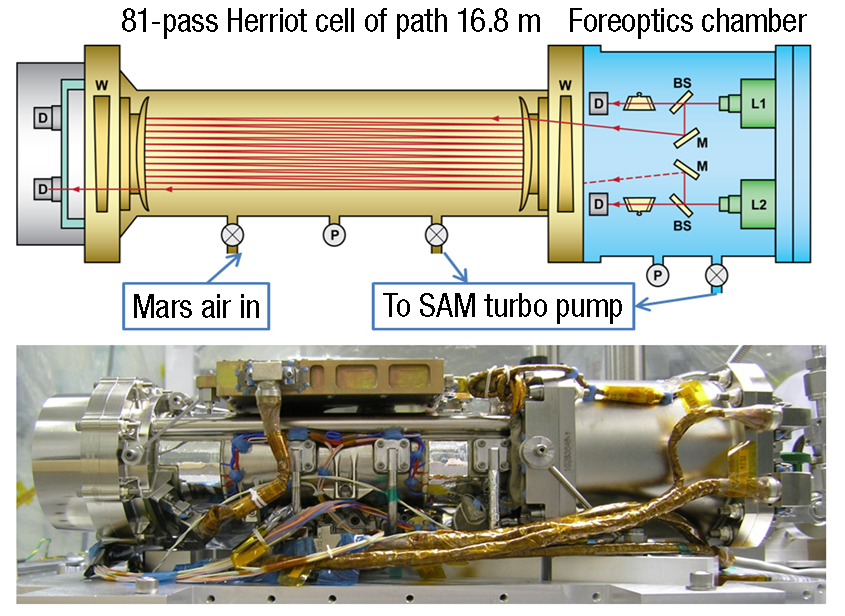
The Tunable Laser Spectrometer on NASA’s Curiosity Mars rover bounces a laser 81 times through a chamber filled with sample gas. This measures absorption of varying laser wavelengths to determine not only concentrations of different gases but also their isotope ratios, which give clues about the sample’s history. Funding and expertise from NASA have helped a company develop a much smaller commercial version of the technology. Credit: NASA
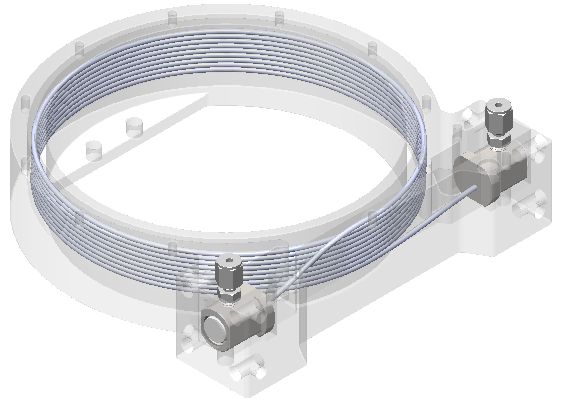

Guiding Photonics’ compact Omega gas cell is a long, hollow, coiled optical fiber. It requires only a tiny gas sample to fill it, but the fiber’s length of 15 feet or more still offers plenty of opportunity for a tunable laser beam to be absorbed by molecules in the sample. Credit: Guiding Photonics LLC

This Guiding Photonics capillary absorption spectrometer is built around the fiber-optic gas cell technology that affiliate company OKSI developed with help from NASA and NOAA. Credit: Guiding Photonics LLC

Nicholas Ward, a researcher with the Department of Energy’s Pacific Northwest National Laboratory, uses an OKSI-developed capillary absorption spectrometer for field measurements of methane in coastal wetlands of Washington state in June of 2021. Credit: Jason Kriesel